About Scitech
Scitech is a not-for-profit organisation proudly supported by the Western Australian Government through the Department of Jobs, Tourism, Science and Innovation
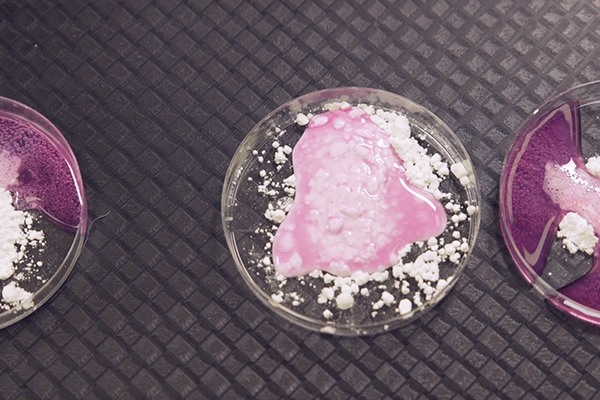
Everyone loves dinosaurs, and this easy chemistry experiment is a great way to engage your kids with science. Using basic household ingredients, discover what happens when you mix an acid and a base together. Can you uncover your dinosaur?
What you’ll need:
Instructions
What you’ll learn
This chemistry experiment demonstrates the reaction between an acid and a base, called a ‘neutralisation reaction’.
The vinegar or citrus juice is acidic, meaning that its molecules have extra hydrogen ions. The bicarbonate soda is basic, which means that its molecules can accept hydrogen ions. When the acid and base are mixed together, the reaction produces water and carbon dioxide gas which we can see as bubbles!
Looking for more activities to do at home?
Upon clicking the "Book Now" or "Buy Gift Card" buttons a new window will open prompting contact information and payment details.